While higher risk class devices have a longer transition period, MDR becomes law for all lower risk class devices, such as bandages and surgical masks, from 21 May 2021. The pressure is on manufacturers to have the technology in place capable of delivering UDI coding that meets these new stringent standards. Fail to do so, you risk not being able to sell your products in the EU.
So, what solution is going to achieve compliant coding? To be fit for purpose, your printer has to deliver on seven fronts.
1. Permanent coding
According to the MDR, every device must display a clearly readable UDI to enable traceability as a qualification for sale in the EU. So it’s critical that these dynamic data barcodes remain clearly legible whatever the conditions the device is transported and stored in. Thanks to edding’s ink expertise, edding printers deliver high resolution, permanent coding; achieving perfect 2D Datamatrix readability even after challenging wash tests.
2. Smart printing
For optimum traceability, the MDR asks for the serialised codes to be printed in both plain text and in machine-readable code, wherever possible. That’s not a problem for edding compact printers. They can overcome the space constraints on device labels with precision printing and a range of print sizes. They also have the technology to combine plain text and 2D barcodes in the same print.
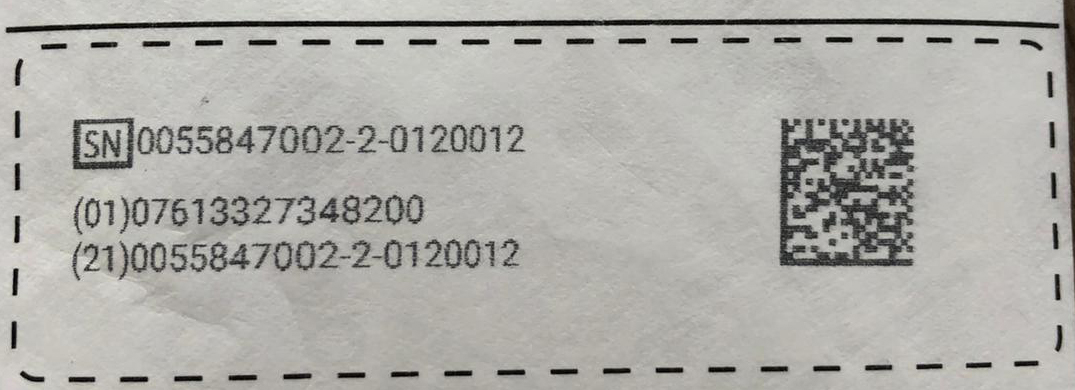
3. Fast and easy information changes
The regulations state that the UDI must always be updated if there are all and any alterations to the device. edding compact printers can handle these serialisation changes quickly and simply. You can update the print information easily via the integrated touchscreen, a web browser or even smartphone. Or alternatively, edding compact printers integrate seamlessly with your network, so changes can be updated automatically from your IT systems.
4. Automated coding for all batch sizes
Typically, many medical device manufacturers buy in labels from a third-party supplier which are then applied in manual or semi-automated processes. But the greater the human interaction with the coding process, the higher the risk of non-compliance. A fully automated process minimises the opportunity for operator error. With edding compact printers, automation is no longer just for the big guys. With their low purchase and running costs and easy-to-use TIJ technology, the edding compact in-line 12 and portable 12 are also ideal for small to medium batch sizes.
5. Operational efficiency
Manufacturers are being challenged by growing competition and rising pricing pressure (driven by the continual squeeze on governments’ healthcare budgets) to boost operational efficiency and strip out production costs. edding compact printers can help. With their clean TIJ technology, they are service and maintenance-free; reducing running costs and maximising production uptime.
6. Futureproof
Invest in a coding solution that can keep pace with the continually evolving regulatory environment for medical devices. For example, countries such as Australia have already stated they are planning to bring their medical device regulations in line with the EU. So manufacturers need to futureproof their production lines with machinery capable of meeting these ever-changing requirements. With the most advanced system architecture on the market, edding compact printers can handle any changes coming down the line, interacting seamlessly with all common network, Manufacturing Control and ERP systems.
7. Stress-free
Finally, the coding solution you buy should offer maximum ease of use. For all medical manufacturers, preparing for the MDR has been a complex, extensive process. Your printer shouldn’t add to that complexity. Built around smart TIJ technology, edding compact printers provide unparalleled ease of use. With the intuitive user interface and menu navigation, anyone can install and begin using the printer in just a few minutes. No technical support or training required.
Contact us to find out more about how edding compact printers can meet your needs.